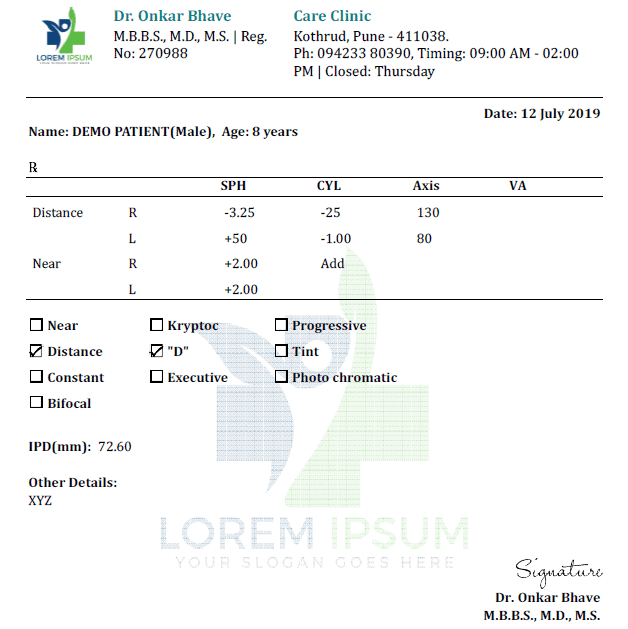
43 medication labels must include
Guidance Document: Labelling of Pharmaceutical Drugs for Human Use 3.8 Labelling of Professional Samples 3.9 Including International Information on Drug Package Labels Claims and Text Content 4.1 Misrepresentation of Classification 4.2 Absence of Ingredients 4.2.1 Sugar-free, Sucrose-free, Sweetener-free 4.2.2 Salt and Sodium-free 4.3 Absence of Side Effects 4.4 Side Effects and Placebo Comparisons What Is a Drug Label? | The Motley Fool Jun 27, 2016 at 11:27PM A drug label refers to all the printed information included with any dietary supplement, over-the-counter medicine, or prescription drug. They're strictly regulated by the...
PDF Labelling of injectable medicines, fluids and lines Label must include the active ingredient, date prepared, volume of lumen and final amount of medicine in units. The label must be placed on the catheter, close to the dressing but not impede the function of the dressing. Labelling of Burettes Burettes must be labelled immediately after an injectable medicine is added, using the state standard pre-printed label for burettes. This label is designed to be peeled off easily at the completion of the

Medication labels must include
› media › 158522Safety Considerations for Container Labels and Carton ... The format and content of prescription drug and biological product labels and labeling must comply with the applicable provisions of the Federal Food, Drug, and Cosmetic Act (FD&C Act) Clinical Labeling of Medicinal Products: EU Clinical Trial ... - ISPE Note: Auxiliary medicinal products should not include concomitant medications, that is medications unrelated to the clinical trial and not relevant for the design of the clinical trial. (CTR (whereas 54)). The Regulation states that IMP and AMP should be appropriately labelled to ensure; subject safety, reliability and robustness of data, and allow the distribution to clinical sites. Pharmaceutical Labeling: Requirements & Guidelines To meet today's FDA regulations, labeling information on drugs must include the following in this order: - Product Name - Drug Facts Table - Active Ingredients - Purpose and Use - Warnings - Directions - Allergic Reactions - Inactive Ingredients
Medication labels must include. A Guide To Veterinary Prescription Label Requirements What Is Required On A Veterinary Prescription Label As shown in the above example, the actual container must include the following information: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's last name How to Read Over-the-Counter and Prescription Drug Labels Some labels include a seventh section with a phone number to call if you have questions or comments. The Drug Facts label for the over-the-counter drug acetaminophen, known by the brand name Tylenol, includes information about ingredients, uses, warnings and directions. Active Ingredient and Purpose. Best practice in the labelling and packaging of medicines Guidance Best practice in the labelling and packaging of medicines This guidance explains the legal framework for labelling and packaging as described in UK legislation and gives best practice for... Drug Labeling - an overview | ScienceDirect Topics drug labeling may contain information on genomic biomarkers that can describe and provide guidance on drug exposure and clinical response variability, mechanisms of drug action, polymorphic drug target, genotype-based dosing, disposition pathways, risk for adverse events, precautions/alerts, drug-drug interactions, contraindications, and …
› AssistMedicationGet ready to assist clients with medication 2. Numbers on medication labels and documents Numbers are everywhere on medication labels and documents. The following medication label and document include examples of information the DSW needs to read and interpret. • Number of tablets • Batch number • Expiry date • Dosage • Age • Temperature • Pregnancy • Barcode ACTIVITY Medicines: packaging, labelling and patient information leaflets Labels must include warnings for safe use of the medicine. All products that contain paracetamol must include statutory warnings. Additional warning statements must be included on the packaging of... sites.northwestern.edu › recalculated › 2019/05/05Calculus in Medicine - Northwestern University May 05, 2019 · In order for doctors to prescribe the correct dosage of a drug and provide a regimen for treatment (ie., “take 2 capsules twice a day”), the drug’s concentration over time must be tracked. This prevents under and over-dosing. The way that a drug’s concentration over time is calculated is using calculus! Ch 8 Pharmacy Flashcards & Practice Test | Quizlet A legal prescription label must include all of the following except: A. Directions for use B. Date the prescription was dispensed C. Name, address, and telephone number of the prescriber ... C. Oral medication reconstitution is a process during which a dehydrated product is changed to a liquid state D. All of the above.
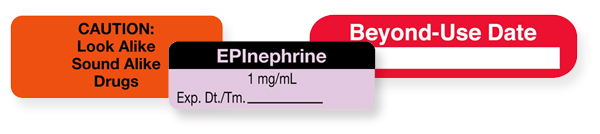
How to Label Prescription Medication for Veterinary Patients A label should include the following components: The name of the veterinary practice, its address, and contact information The veterinarian's name, the patient's name and species, and the client's... Guidelines for Labeling Pharmaceutical & Healthcare Products Several important things to include on a pharmaceutical or healthcare product label: 3. Formatting Labels for FDA Approval. Your labels must be designed in the appropriate FDA format for your product's classification like OTC medications, oral contraceptives, combination products, etc. Click here for a list of labeling guides relating to drugs. Safe Labeling Helps Prevent OR Medication Errors Label information must include a medication's name and strength as well as amount when medications are mixed (as with antibiotic irrigations, tumescent and heparin solutions, and epinephrine). The unit of measure — percent, grams, milliliters, or units — must be recorded along with the date the medication is prepared. How to read prescription drug labels - BeMedwise Whenever you are prescribed a medication, you should read and follow the information in the medication's "label" in order to ensure your safety. All prescription medicine containers include information on the label including the patient's name, the name of the medicine, dosage and instructions on how often to take the medicine.
What's on a prescription label? - Knowledge is the best medicine Rollover A-K below to see the various part of a prescription label. * A Drug Identification Number (DIN) is an eight digit number assigned by Health Canada to a drug product prior to being marketed in Canada. It uniquely identifies all drug products sold in a dosage form in Canada and is located on the label of prescription and over-the-counter ...
Medicine labels: Guidance on TGO 91 and TGO 92: 1. Using the Orders ... Your medicine label must include the medicine's: batch number; expiry date. Each of these must be immediately preceded by a relevant prefix and examples are provided in section 6 of both Orders. Prefixes that cannot be used for expiry dates are also identified in section 6.
Pharmacology Chapter 5 (Prescriptions and Labels) - Quizlet -the strength of the medication-the dosage form-the quantity (how many to dispense)-the manufacturer (if the drug is generic)-refill information must also be included-name of the prescribing physician and license classification (MD or FNP)-pharmacist adds the date when the medication will expire or lose its potency and should be discarded
› article › thyroid-medicationThyroid Medication Mistakes You Don't Want to Make Jan 12, 2019 · Thyroid hormone replacement medications treat hypothyroidism.The common drugs include levothyroxine (Synthroid, Levoxyl, Tirosint), liothyronine (Cytomel, compounded time-released T3), and natural ...
Medication Safety & Labeling | USP The labeling section of chapter <1> was revised to include prevention of medication errors related to misinterpreting messages on ferrules and cap overseals. Healthcare practitioners using injectable products must be able to easily see and act on labeling statements that convey important safety messages critical for the prevention of imminent life-threatening situations.
The Over-the-Counter Medicine Label: Take a Look | FDA All nonprescription, over-the-counter (OTC) medicine labels have detailed usage and warning information so consumers can properly choose and use the products. Below is an example of what the OTC...
FDA's Labeling Resources for Human Prescription Drugs Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,...
Product-information requirements | European Medicines Agency The European Medicines Agency (EMA) provides guidance and templates to provide marketing authorisation applicants with practical advice on how to draw up the product information for human medicines, which includes the summary of product characteristics, labelling and package leaflet.. EMA's guidance explains the content that should be included in these documents, as well as standard headings ...
› resources › safety-enhancements-everySafety Enhancements Every Hospital Must Consider in Wake of ... Place auxiliary labels on all storage locations and/or ADC pockets/ drawers/lids that contain neuromuscular blockers that clearly warn that respiratory paralysis will occur, and ventilation is required (e.g., “WARNING: CAUSES RESPIRATORY ARREST—PATIENT MUST BE VENTILATED”). The warning should be visible when ADC pockets/drawers/lids are open.
4. Documenting Medications (MAR). | Aplmed Academy Each medication must be documented at the time of administration. For example, if eight medications are administered the QMAP must initial the MAR eight times indicating that each medication has been administered, refused or unavailable. ... Labeling requirements for over-the-counter (OTC) medications include in the original manufacturer's ...
A Primer on Pharmaceutical Label Types and Requirements | Luminer A professional package insert (sometimes known as a PPI) is an essential part of any pharmaceutical label design. It's the most basic and important way to express information about the drug in question to both patients and the individual (s) prescribing or recommending it. It includes the official description of the drug itself, what it's ...
en.wikipedia.org › wiki › MedicationMedication - Wikipedia A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. [1] [2] Drug therapy ( pharmacotherapy ) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate ...
quizlet.com › 372920952 › chapter-53-medicationChapter 53. Medication Administration Flashcards | Quizlet When administering medications, the medical assistant must observe the rights of medication administration (see Table 53-1) to avoid errors and ensure patient safety. The six basic rights of medication administration include right patient, right drug, right dose, right route, right time, and right documentation.
Understanding Drug Labels | Basicmedical Key The epinephrine injection label ( Fig. 5.6) indicates a dosage supply of 0.1 mg/mL, and the total volume of the ampul is 10 mL. Figure 5.4 The dosage strength of this dosage form of Diflucan ® (fluconazole) is 200 mg. Figure 5.5 The dosage strength of this drug is 125 mg (200,000 units) penicillin V in 5 mL.
Drug labeling, Information about Drug labeling - FAQs The label must describe the uses of the medicine along with the conditions under which the medicine should not be used. The consumer must be given directions for the contraindications; for example, "Talk to your health care professional before takingthis medication if any of these apply to you." Under FDA regulations, the label must describe foods, drugs and activities that the patient should avoid while taking the medication, along with any related precautions.
Over the Counter (OTC) Drug Labels - Poison All OTC drug labels include "Drug Facts", the who, what, how, when, and why of that medicine. The Drug Facts tell you what you need to know to give the right drug, in the rightdose, to the right person, at the right time, in the right way, and for the right purpose.












Post a Comment for "43 medication labels must include"