41 cautionary and advisory labels for medicines
Medicine labels - what do the warning statements mean? | Health ... Some medicines can cause stomach problems, indigestion and problems with your throat and oesophagus (the pipe between your throat and stomach) if they are not swallowed properly. Taking them with a large glass of water helps to wash them down. Take each dose on an empty stomach - one hour before or two hours after food. Label Statements Database - Medsafe This database lists the warning and advisory statements that are required on medicine and related product labels under regulations 13 (1) (i) and 14 (1) (f) of the Medicines Regulations 1984. Words of a similar meaning to the statements in the database may be used and individual statements may be combined provided the intent is not changed.
StirlingFildes | HealthCare Cautionary Advisory Labels The StirlingFildes Cautionary Advisory Label (CALs) system produced by the PSA is a valuable labelling system designed for helping pharmacists to fulfil their legal and professional obligations, promote quality use of medicines, and provide optimal outcomes for consumers.
Cautionary and advisory labels for medicines
Outcome of the consultation on the proposed warning and advisory ... The Label Statements Database will be updated on 1 September 2022. Medicines released for supply in New Zealand after 1 March 2024 must have updated package labels that include the new warning and advisory statements. However, we encourage sponsors to update their labels before this date, if feasible. StirlingFildes | Printing, Packaging and Consumables Cautionary & Advisory Labels. Buy a mixture of 12 or more labels and save. The savings will be calculated on your invoice. Product Image Product Description & Code Product Details; Warning Label No. 1 Code: WARN 1: 1,000 per box 46 x 16mm: Warning Label No. 1A Code: WARN 1A: Cautionary and advisory labels for medicines - Everything2.com These are the code numbers and their meanings for the cautionary labels used by pharmacist s when dispensing medicine s in the UK. Extra counselling may be given with relation to age, experience , background and understanding of the patient. Warning. May cause drowsiness
Cautionary and advisory labels for medicines. PDF Revisions to APF24 Cautionary advisory labels Revisions to Table A2. Recommended cautionary advisory labels for medicines Revised CAL recommendations for the following medicines are shown in the table below. Medicine Revised ancillary label (number) and/or additional instruction (letter) Abacavir Oral solution: 7b (60 days), 12†, 21 Tablet: 12†, 21, A Aciclovir Eye ointment: 7b (28 ... Cautionary and advisory labels for medicines - SlideShare Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counseling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the patient needs to know. Kiran Sharma, KIET School of Pharmacy 11/12/2013 2 Cautionary advisory labels - Australian Pharmacist The dispensing pharmacist has dispensed the patient's discharge medications and has labelled simvastatin with cautionary advisory label 21 (Special handling and disposal required - ask your pharmacist) and label A (Swallow whole do not crush or chew). You are concerned that these labels may alarm and/or confuse the patient. What should you do? PDF Revisions to APF25 Cautionary advisory labels - psa.org.au Cautionary advisory labels Revisions to Table 2.2 Recommended cautionary advisory labels for medicines Revised CAL recommendations for the following medicines are shown in the table below. Medicine Revised ancillary label (number) and/or additional instruction (letter) Acetazolamide 8, 10a, 12 Adalimumab 6 (except syringe in use), 7b*, 13
Guidance for cautionary and advisory labels | About | BNF | NICE Wordings which can be given as separate warnings are labels 1-19, 29-30, and 32. Wordings which can be incorporated in an appropriate position in the directions for dosage or administration are labels 21-28. A label has been omitted for number 20; labels 31 and 33 no longer apply to any medicines in the BNF and have therefore been deleted. DOCX Required advisory statements for medicine labels For example, the entry for Alclometasone shows the condition "When included in Schedule 3 of the SUSDP" in Column 2. Medicines included in Schedule 3 of the SUSDP that contain this ingredient must include the advisory statements numbered 2, 80, 112, 117, 52 on the label and meet the additional requirement 'j' in relation to all of the required statements. Cautionary_and_advisory_label - chemeurope.com Standard cautionary and advisory labels offer advice but are not exhaustive. The labels are not a substitute for adequate counselling by prescribers and dispensers (most medicines are dispensed by pharmacists) but are intended to reinforce essential information the patient needs to know. Label Wording and Warnings Safer dispensing labels for prescription medicines There is an emerging consensus for communicating less confusing, more informative and safer information on dispensing labels. 7-9 These labels have been called patient-centred labels, and recommendations have been developed based on research in health literacy and health communication (see Box 1). 3,10,11 Advice includes: use larger font sizes (e.g. 12 point and above)
PDF Improving the safety and quality of pharmacy dispensing labels dispensing label and that through redesigning the dispensing label, cautionary and advisory statements could be appropriately incorporated. It was noted that including cautionary and advisory statements would require a larger label than that currently used. 2 United States Pharmacopeia. 2012. USP-NF General Chapter 17 -Prescription Container ... Cautionary Advisory Labels (CAL) - Medi Print Cautionary Advisory Labels (CAL) Categories. Laser Labels. Laser Sheets; Show All Laser Labels. Drug Labels . Burette & Additive Labels ; Drug Identification Labels ; ... This medication may cause drowsiness - CAL Code: CAL-01 Size: 16 x 46mm Quantity: 1,000 per.. Add to Cart. 50 Common Warning Labels On Medication Containers Top 50 Common Warning Labels and Their Meanings The medication must be swallowed whole. Because certain drugs are designed to be either fast-acting or slow-releasing, damaging the outer coating may lead to harmful damages to the body. The medication is intended for external use only. Ingesting it may lead to undesirable effects or even poisoning. Bagot Press - Pharmacy Consumables & Print Specialists PHARMACEUTICAL > Cautionary and Advisory Labels The Bagot Press Cautionary and Advisory label system is one of the few approved licences used for printing labels for prescribed medicines and the bright colour labels draw attention to important specific information to patients. Ordering Guide
PDF cautionary advisory labels - Openbook Howden cautionary advisory labels OBH 18642 CAL's are a valuable tool for helping pharmacists to fulfil their legal and professional obligations, promote quality use of medicines, and provide optimal outcomes for consumers. Reproduced with the permission of the Pharmaceutical Society of Australia. Sold in dispenser boxes of 1000.
Labelling of the pharmaceuticals - SlideShare INTRODUCTION • Label means display of printed information which is securely affixed to the containers and primary packaging containing the medicines. • Label should provide the patient with all necessary info. for appropriate use of medicine - General labeling requirements - Cautionary and advisory labels - Special instructions for ...
Cautionary and advisory labels | About | BNF | NICE To be used on preparations containing ofloxacin and some other quinolones, doxycycline, lymecycline, minocycline, and penicillamine. These drugs chelate calcium, iron, and zinc and are less well absorbed when taken with calcium-containing antacids or preparations containing iron or zinc.
New label advice for antibiotics - NPS MedicineWise Find out more about the antiviral medicines helping to treat COVID-19. Medicine Finder. Find information on medicines by active ingredient or brand name. Keep a medicines list. ... In consultation with other organisations, the Pharmaceutical Society of Australia has revised the cautionary advisory label used by pharmacists for antibiotics. It ...
Required Advisory Statements for Medicine Labels (RASML) Australian labelling requirements for non-prescription medicines ( Therapeutic Goods Order No. 92) require some over-the-counter and complementary medicine labels to contain particular warning statements ('advisory statements') about specific risks related to use of the medicines.
New Label 24 to help pharmacists reduce opioid risks The CAL is recommended for opioid medicines, including buprenorphine, codeine, dihydrocodeine, fentanyl, hydromorphone, methadone, morphine, oxycodone, tapentadol, and tramadol. Pharmacists are advised to provide the Opioid medicines patient information handout to any patient receiving opioid medicine that carries Label 24.
Labels on medicines and poisons - Department of Health Labels on medicines and poisons All medicines and poisons containers must be labelled so as to clearly identify the contents. This is important to prevent inadvertent consumption and poisoning. Labels must meet uniform Australian standards on text size, language and warnings. Labels on poisons Dispensing labels Labels on medicines More information
Auxiliary label - Wikipedia An auxiliary label (also called cautionary and advisory label or prescription drug warning label) is a label added on to a dispensed medication package by a pharmacist in addition to the usual prescription label. These labels are intended to provide supplementary information regarding safe administration, use, and storage of the medication. [1]
PDF National standard for labelling - Safety and Quality 3.6 Cautionary advisory labels 16 4 Standards for dispensed medicine labels 17 Standard 1: Prominently display the information that consumers need to take their medicine ... The dispensed medicine label provides customised information about the medicine and how the consumer should use it, at the point of use. The label is especially important ...
Cautionary And Advisory Labels - Medico Pak Cautionary And Advisory Labels. These labels are available in eye-catching fluro orange with different statements or instructions. We recommend using these labels to assist care facilities to maximise the safety by affixing appropriate labels to the pack where necessary. ... As Required Medication: 200: 38071: Look For A Second Pack: 200: 38072 ...
Cautionary and advisory labels for medicines - Everything2.com These are the code numbers and their meanings for the cautionary labels used by pharmacist s when dispensing medicine s in the UK. Extra counselling may be given with relation to age, experience , background and understanding of the patient. Warning. May cause drowsiness
StirlingFildes | Printing, Packaging and Consumables Cautionary & Advisory Labels. Buy a mixture of 12 or more labels and save. The savings will be calculated on your invoice. Product Image Product Description & Code Product Details; Warning Label No. 1 Code: WARN 1: 1,000 per box 46 x 16mm: Warning Label No. 1A Code: WARN 1A:
Outcome of the consultation on the proposed warning and advisory ... The Label Statements Database will be updated on 1 September 2022. Medicines released for supply in New Zealand after 1 March 2024 must have updated package labels that include the new warning and advisory statements. However, we encourage sponsors to update their labels before this date, if feasible.








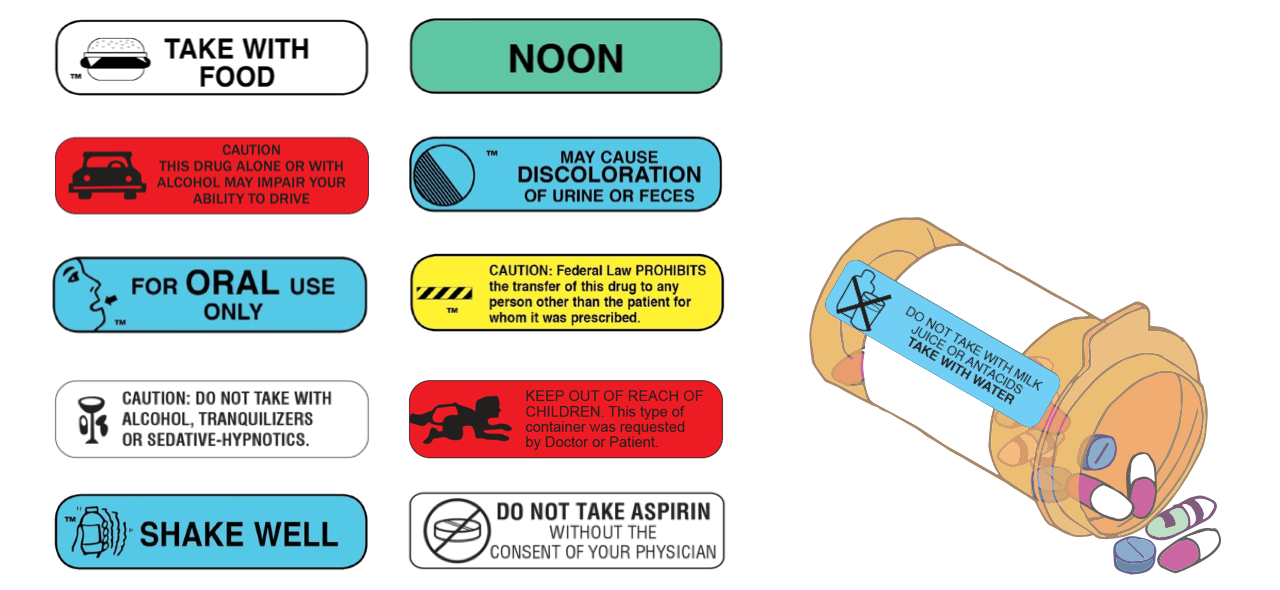










![PDF] Improving prescription drug warnings to promote patient ...](https://d3i71xaburhd42.cloudfront.net/e087ba4ebaa8ed043bf22bd2ab63292d6a05b9d4/2-Figure1-1.png)











Post a Comment for "41 cautionary and advisory labels for medicines"